Cross-Disciplinary Collaboration with Photonics is Key to Advancing the Medical Field

Introducing new medical devices into the healthcare system is no easy task. This is not only due to strict regulations but also because medical professionals need extensive training on new technologies—a process that can take several years.
Despite these challenges, medical professionals are increasingly open to adopting technologies like photonics to address everyday challenges. As emphasized during the EPIC Meeting at ICFO, cross-disciplinary collaboration is not just beneficial but essential for progress.
Introducing new medical devices into the healthcare system is a challenging endeavor. This is not only due to strict regulations but also because medical professionals require extensive training on new technologies—a process that can take several years.
Despite these challenges, medical professionals are increasingly open to adopting technologies like photonics to address everyday challenges. This was a key theme emphasized during the EPIC Meeting at ICFO, where cross-disciplinary collaboration was highlighted as not just beneficial but essential for progress.
The agenda for this meeting was collaboratively curated by the EPIC and ICFO teams. ICFO, a member of the Barcelona Medical Photonics Network, fosters research and development in photonics through partnerships with several biomedical and clinical organizations. This collaboration provided insights into ongoing projects at Hospital Clínic de Barcelona, Hospital Sant Joan de Déu de Barcelona, and IDIBELL.

Below is a summary of some of the presentations delivered during this two-day meeting held at the ICFO facilities.
SESSION 1 – PHOTONICS ASSISTED CANCER PATHOLOGY
Non-Invasive Diagnosis of Skin Cancer –Professor Josep Malvehy, MD – Dermatologist at Hospital Clinic
In recent decades, multiple non-invasive methods for evaluating skin tumors have been integrated into clinical dermatology practice, significantly enhancing early diagnosis and treatment outcomes. Dermoscopy remains a cornerstone in the diagnosis of melanocytic and epithelial carcinomas, with growing applications for inflammatory and other dermatological conditions. For patients at high risk of melanoma, such as those with atypical mole syndrome, digital dermoscopy and total body photography (TBP) play a pivotal role in detecting new lesions and monitoring changes in existing ones—key indicators of malignancy. Recent advances in digital imaging, including high-resolution 2D and 3D TBP devices with polarized light, have further improved the speed and accuracy of lesion assessment.
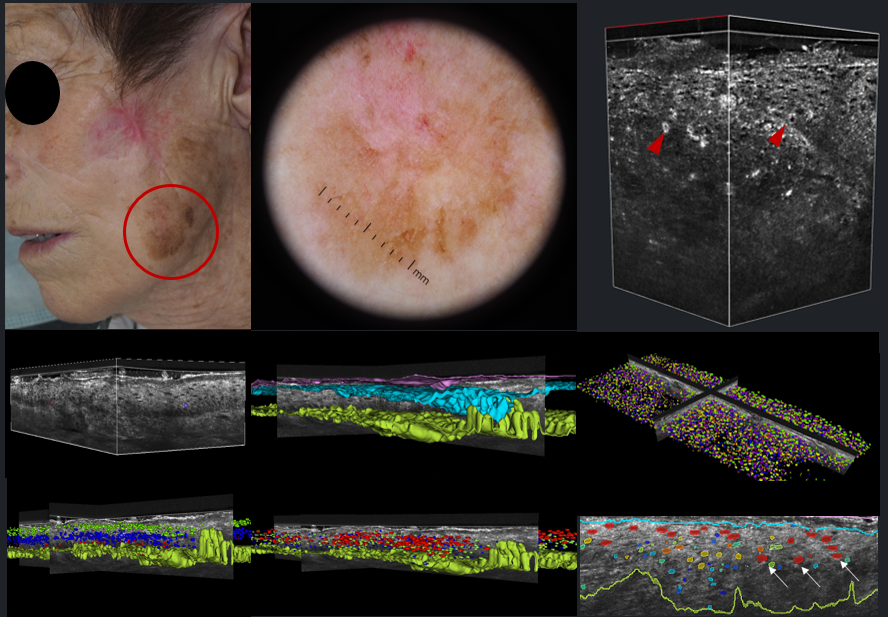
Alongside dermoscopy, optical techniques such as in vivo and ex-vivo confocal microscopy (RCM), optical coherence tomography (OCT), and line-field OCT have expanded the diagnostic toolkit. These technologies offer unprecedented imaging capabilities, allowing for detailed analysis of skin structure at the cellular level. Additionally, the integration of artificial intelligence (AI) for automated image classification and tumor detection is emerging as a promising approach to further refine diagnostic accuracy.
In his presentation, Professor Malvehy explored the integration of these advanced imaging technologies with clinical data, pathology, genetics, and molecular biology, aiming to facilitate deep phenotyping of skin cancer. Such a multi-faceted approach promises to improve patient risk stratification and enhance the detection of malignant skin tumors, ultimately advancing personalized care in dermatology.
Intraoperatory Tumour Assessment with Raman Spectroscopy, including regulatory Aspects. ByIwan Schie, Professor for Biomedical Engineering at Leibniz Institute of Photonic Technology
The presentation highlighted the potential of Raman spectroscopy as a label-free optical method for the detection and characterization of cancerous tissue across various anatomical regions, including bladder, gastrointestinal, and head and neck cancers. The study emphasizes the transition from ex vivo validation to in vivo applications, demonstrating the feasibility of integrating a Raman-based diagnostic system ‘invaScope’ into clinical workflow.

Using a minimally invasive endoscopic setup, Raman spectra were captured from healthy and tumorous tissues during clinical procedures, correlating the results with standard histopathological evaluations. The research also addresses the regulatory framework within the European Medical Device Regulation (MDR2017/745), detailing the necessary steps for clinical investigation approval. Preliminary results from the study underscore the diagnostic accuracy and clinical applicability of Raman spectroscopy in real-time cancer detection, presenting it as a promising tool for improving early diagnosis and personalized treatment strategies.
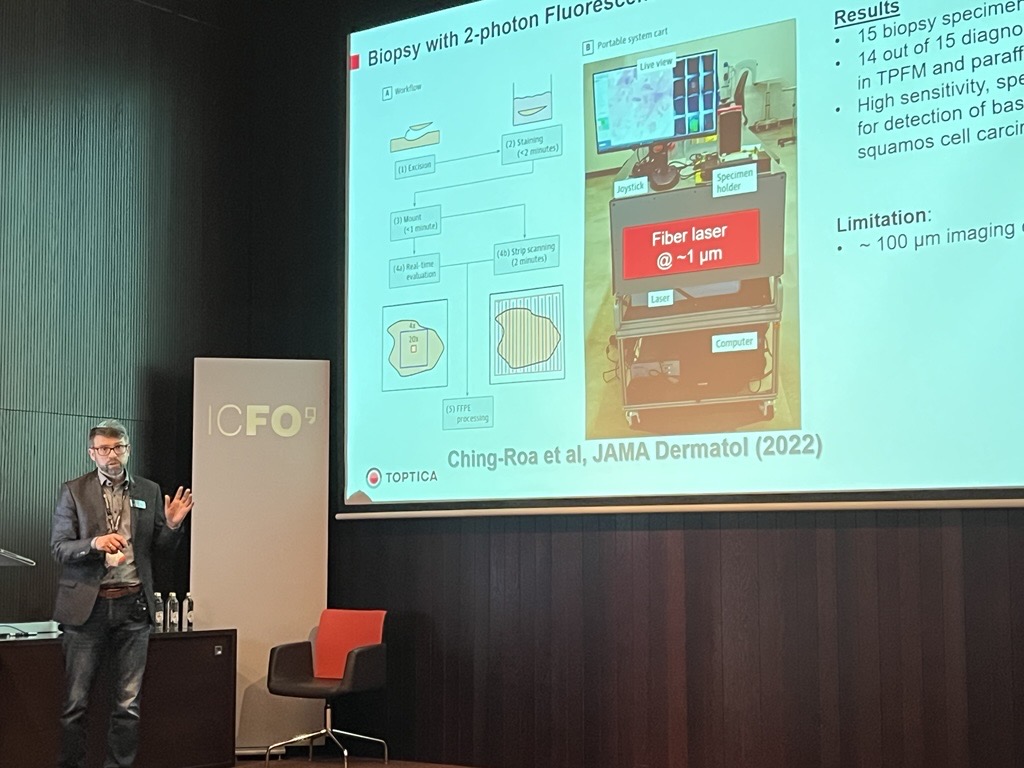
Non-linear Fluorescence Imaging to Improve Skin Cancer Diagnosis. By Alexander Jelzow, Sales Manager at TOPTICA Photonics
Nonmelanoma skin cancer (NMSC) is primarily diagnosed by histologic analysis of skin biopsy specimens in paraffin embedded or frozen sections, which takes days to weeks before a formal diagnosis is made. Two-photon fluorescence microscopy (TPFM) with compact femtosecond fiber lasers has the potential for point-of-care diagnosis of NMSC and other dermatologic conditions, which could allow diagnosis and treatment at the same site.
SESSION 2 – PHOTONICS-GUIDED MONITORING AND THERAPY FOR ONCOLOGICAL DISEASES
A Toolbox for the Study, Optimization and Personalization of Cancer Therapies. By Clara Vilches, Researcher Medical Optics at ICFO
Despite the advancements in diagnostics and treatments made over the years, cancer is still one of the leading causes of death worldwide, evidencing a real clinical need for a more precise cancer management. In the last decades, several nanomedicine and light-activated therapies, such as photodynamic or plasmonic photothermal therapy, have emerged as promising complimentary techniques to improve current standard cancer treatments.

However, as in other treatment approaches, the complex tumor heterogeneity remains a challenge in the development of these therapies and it demands for tools to accomplish the goals of precision medicine. In this talk, we will see how diffuse optical techniques, which offer non-invasive means to monitor tissue hemodynamics, tissue physiology and drug concentration, could play a defining role in the optimization and personalization of cancer therapies.
Fluorescence Guided Surgery. By Muriel Abbaci, Group Leader, Surgery and Pathology Photonic Imaging –SP2I at Gustave Roussy
In her presentation, Muriel Abbaci provided an overview of various clinical studies conducted in the operating room setting for diverse indications. SP2I works closely with academic and industrial partners to design and conduct clinical protocols, to evaluate the effectiveness of new technologies and transfer them to the clinic. Their goal is to provide Gustave Roussy (GR) patients with tailored, precision treatments to improve surgical outcomes.

The GR provides care for over 49,000 patients annually. GR’s unique model integrates research and clinical care, with 40% of patients benefiting from innovative treatments through clinical trials. With around 20 surgeons on site, the department performs approximately 4,400 surgeries per year. The surgery and pathology photonic imaging group at GR has been developing and transferring innovative photonic imaging techniques for 16 years to improve patient care in surgery and pathology. Building on a solid experience (1300 patients included), SP2I aims to develop precision diagnostic and therapeutic tools.
Intra-operative Assessment of Surgical Margins and Sentinel Lymph Nodes by Fast Raman Spectroscopy. By Ioan Notingher, Professor of Physics, Biophotonics Group at University of Nottingham
Raman spectroscopy is a powerful technique for analysing biological tissues at a microscopic level. It has high molecular specificity and is non-destructive, but is a low light technique requiring long acquisition times for imaging. This limitation can be overcome by combining Raman spectroscopy with faster but less specific imaging techniques, such as auto-fluorescence (AF) imaging. This dual-modality approach enables fast analysis of tissue specimens (within 20-30 minutes) with high sensitivity and specificity, generating objective diagnosis results that can be acted upon in the clinic.

During his talk, Ioan presented the latest results on clinical integration and diagnostic accuracy studies. The focus of the presentation is on intra-operative assessment of sentinel lymph nodes during breast cancer surgery and detection of incomplete surgical margins during Mohs micrographic surgery of basal cell carcinoma.
Cancer depth assessment using Optical Coherence Tomography. By Maaike de Jong, Chief Medical Officer of Scinvivo
Worldwide, the incidence of cancer is growing, as life expectancy increases. Current diagnostic imaging tools are often not suitable to provide the required information for correct and complete diagnosis. In particular, information on the tissue structure beyond the surface at high resolution is missing. With Scinvivo’s imaging platform doctors will be able to visualise both the superficial and the profound tissue structure in real time during a standard endoscopic examination, using optical coherence tomography. An incredible resolution of only a few micrometers, allows the detection of tumors very early.
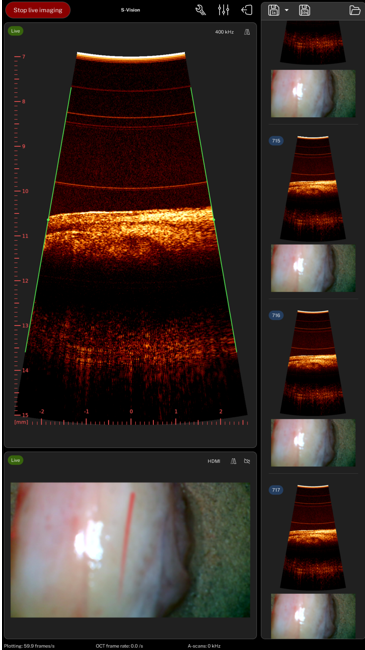
This will make cancer treatment radically more accurate, faster. The first use case of this technology is bladder cancer (BC). Due to the high recurrence rate of BC, it is the most costly cancer from diagnosis to death. BC treatment boils down to getting insight into the bladder wall structure to determine the stage of the tumor growth. With the imaging tools currently available this is not easily done and leads to unnecessary surgeries and removal of bladders. Scinvivo’s imaging platform will save lives, personalise treatment options, increase the quality of the patients’ lives and reduce health care costs.
Hyperspectral imaging (HSI) in the Visible (VIS) and Short-Wave InfraRed (SWIR) on freshly resected breast cancer specimens. By Fabrizio Preda, CEO of NIREOS
In 2021, more than 270000 new cases of breast cancer were diagnosed in the United States among women. Prevention and early screening are key in the fight against breast cancer but, in some cases, surgery is the only viable option. To minimize the risk of recurrence of the cancer after the surgery, intraoperative tumoral margin assessment is crucial. The surgeon identifies the size of the tumor and its location through palpation and visual inspection. This makes the process subjective and its effectiveness dependent on the experience of the surgeon. Effective coordination with the pathologist is essential to confirm that the margins are clear of cancerous cells, yet this collaboration is challenging due to the time-sensitive nature of the procedure. Thus, there is a strong interest in exploring new methodologies that can assist the surgeon in the intraoperative assessment of tumor margins in a more objective, reliable and time efficient manner.
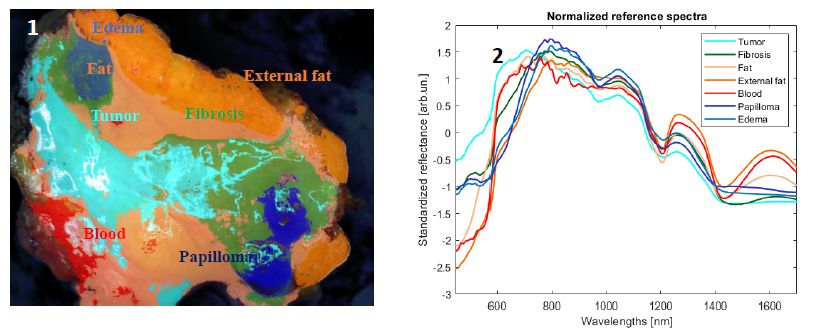
Several studies have recently tried to leverage hyperspectral imaging (HSI) as a tool for the identification of tumoral areas within surgical specimens1,2. Absorption peaks of important biological chromophores such as water, lipids, hemoglobin and 𝛽-carotene are present in the visible (VIS) and short-wave infrared (SWIR) spectral regions. As tumoral tissue and healthy fat tissue have different chemical compositions, spectra act as a fingerprint of the tissue under examination. In order to investigate this, we performed a preliminary clinical study using a staring hyperspectral camera at macro resolution working in the visible-short wave infrared (VIS-SWIR) region on freshly resected breast cancer. Measurements were taken in the department of pathological anatomy of the Istituti Clinici Scientifici Maugeri located in Pavia, Italy.
Fig. 1 shows a classification based on the Spectral Angle Mapper (SAM) algorithm. Average standard noise variate (SNV) corrected spectra of endmembers for each class are shown in fig.2. Significant differences are visible below 600 nm and around the absorption peaks of water and lipids in the SWIR. By comparing the hyperspectral image with the corresponding hematoxylin and eosin (H&E) stained image, it is possible to objectively associate a label to each spectrum and identify the possible presence of tumor at the margins. In the future, this technology could help surgeons assess the location of residual tumor and improve the intraoperative surgical procedure.
SESSION 3 – EMERGENT PHOTONICS TECHNOLOGIES FOR OTHER MEDICAL APPLICATIONS
Medical Diagnosis of Drug Allergy Detection. By Eduardo Martínez Castellano, Physicist and Medical Doctor at Bioherent
The identification of new and disease-specific biomarker panels is seeing an unprecedented growth, hand in hand with the irruption of new AI technologies. However, the transference of much of these advances to clinical practice is still limited by the accessibility of techniques such as immunoassays, with scarce availability outside of hospital central laboratories.

Integrated photonics technology, external up to recent years to the biomedical field, is called to revolutionize in-vitro diagnostics. Coherent-readout interferometry has demonstrated unprecedented sensitivity and ultra-low limits of detection, which allows the identification of biomarkers that are inaccessible to traditional immunoassays. In addition to this, photonic chips can be easily integrated into fast, low-cost and fully automated devices with potential to decentralize molecular techniques.
Non-invasive Blood Glucose Measurement by Mid-infrared Spectroscopy: Principle and Validation. By Werner Mäntele, Chief Scientific Officer (CSO) at DiaMonTech
DiaMonTech in Berlin (Germany) founded in 2015 has introduced a technology for non-invasive glucose measurement (NIGM) for diabetes patients. DiaMonTech‘s NIGM technology targets glucose molecules in interstitial fluid (ISF). An infrared beam from a quantum cascade laser (QCL) tuned to wavelengths between 8 and 12 µm where glucose has a highly specific fingerprint absorbance selectively excites glucose molecules in skin. Absorption results in a small amount of heat in skin detected on the surface with a proprietary photothermal deflection technique.

This technology is painless, harmless, and does not require consumables. Based on previous R&D work at Frankfurt University, DiaMonTech has developed a table-top NIGM device for clinical studies (“D-Base“) and currently completes a hand-held device (“D-Pocket“) the size of a smartphone. DiaMonTech has started further miniaturization towards integration of the technology into a smart watch.
Narrow or broadband LEDs as specific light sources in various medical applications. By Antje Thamm VP Sales & Marketing at EPIGAP OSA
LEDs of different wavelengths in the red and infrared range are used today in various sensors for medical applications. Even with a low light output of just a few milliwatts, vital parameters can be precisely determined via the transmission through the skin and the absorption of light in tissue and blood. This is particularly important in the continuous monitoring of premature babies, whose oxygen content in the brain can be precisely monitored using a new, miniaturized LED-based sensor. This is essential as a lack of oxygen has a negative effect and leads to the death of brain cells, while an overdose acts like a cell poison and can lead to blindness in patients.
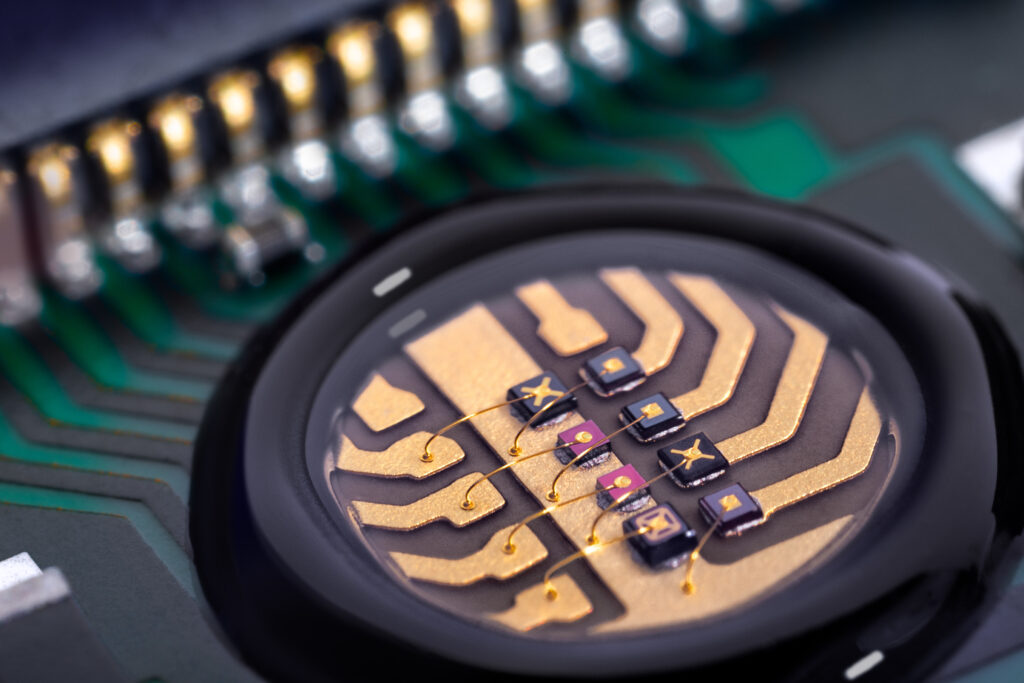
The presentation of Antje provided insights into the development and construction of the illumination in such sensors. Other medical applications require good illumination in order to reliably detect vital tissue during operations. A high-performance broadband LED supports multispectral imaging to improve contrasts and thus the recognizability of the tissue condition and structure. Specific application examples are presented.
High-power Compact VCSEL Subsystem as Game-changer. By Patrick Leisching, CTO at iThera Medical
The presentation reviewed photonic systems (LD/LED/VCSEL/SSL) used for photoacoustic imaging and identify the major challenges to propel this technology into routine clinical use: a factor of 100 in cost, volume and power consumption for photon generation must be reached. The next-gen photonic subsystem presented is based on multi-junction VCSEL arrays and bare ToF CMOS driver chips.
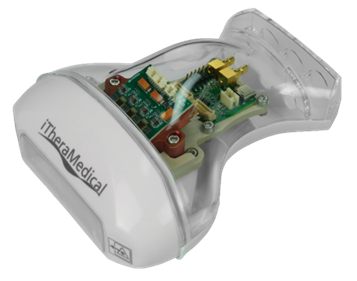
This VCSEL-based approach can replace current photonic systems. In addition, the concept is scalable and paves the way towards future patch and sensor photoacoustic applications.
Company visits: Sensofar, Sant Joan de Déu and BetterCare
Barcelona Children’s Hospital Sant Joan de Déu, SENSOFAR, and Better Care were included in the optional agenda of the meeting. At SENSOFAR participants could visit the facilities of this optical metrology provider specialized in high-precision 3D surface measurement systems that integrate advanced photonic technologies.



At the Barcelona Children’s Hospital Sant Joan de Déu, EPIC members could see the impact of photonics in the daily care of the patients. They visited the Clinical Command Center, CORTEX, a state-of-the-art control centre that enables real-time evaluation of key data on the hospital’s performance and improved management of resources and beds.



And finally they visited Better Care, a provider of digital health solutions, leveraging photonics-based monitoring systems to enhance patient care and clinical decision-making.



The EPIC Meetings always provide networking opportunities during coffee breaks, as well as at lunch and dinner receptions. Below is a selection of pictures—click here to view the full album.






Next year, we will meet at the Gustave Roussy (GR) Center to discuss advancements in cancer therapy and surgery. Gustave Roussy provides care to over 49,000 patients annually. For the past 16 years, the surgery and pathology photonic imaging group at GR has been developing and implementing innovative photonic imaging techniques to enhance patient care in surgery and pathology. Their mission is to deliver tailored, precision treatments to improve surgical outcomes for Gustave Roussy patients.
For more information and to register for the meeting, please click here.